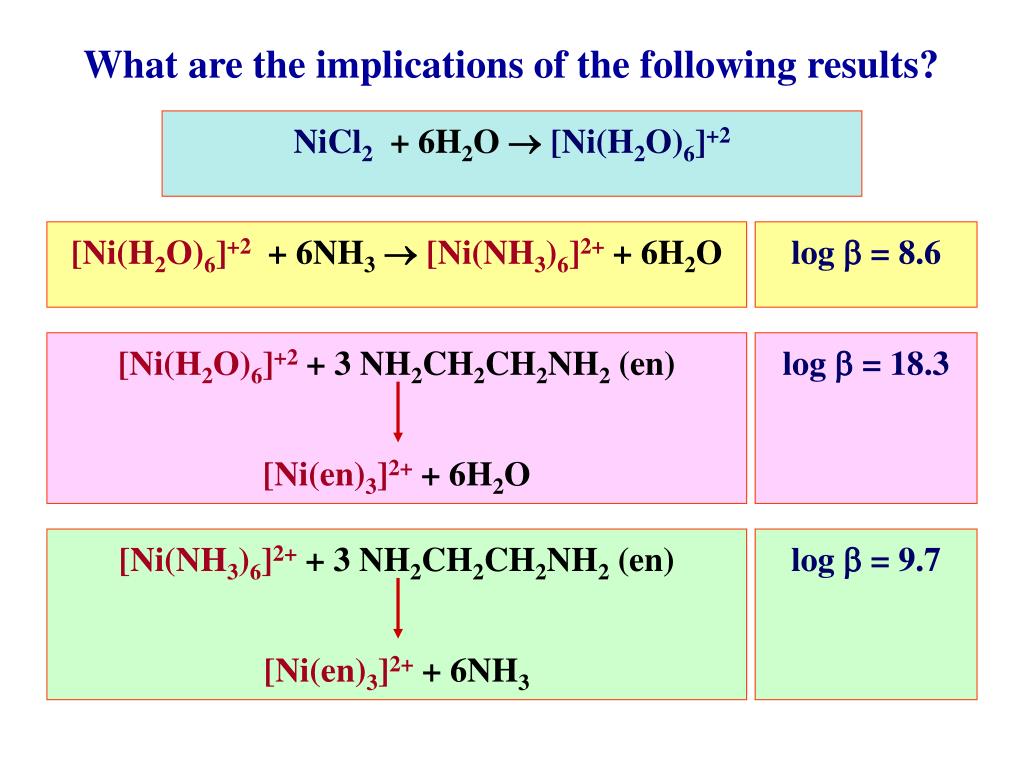
a. [Ni(H2O)6]^2+ (aq) is green in colour whereas [Ni(H2O)4 (en)^2+ (aq)is blue in colour , give reason in support of your answer . - Sarthaks eConnect | Largest Online Education Community
![Studied conformations of [Ti(H2O)6] 3+ and [V(H2O)6] 3+ with planarly... | Download Scientific Diagram Studied conformations of [Ti(H2O)6] 3+ and [V(H2O)6] 3+ with planarly... | Download Scientific Diagram](https://www.researchgate.net/profile/Roland-Meier-3/publication/244410144/figure/fig1/AS:1041086483017728@1625225768337/Studied-conformations-of-TiH2O6-3-and-VH2O6-3-with-planarly-ligating-water_Q320.jpg)
Studied conformations of [Ti(H2O)6] 3+ and [V(H2O)6] 3+ with planarly... | Download Scientific Diagram
![IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O)6 ](SO4)2 IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O)6 ](SO4)2](https://journals.iucr.org/e/issues/2020/03/00/xi2016/xi2016scheme1.gif)
IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O)6 ](SO4)2

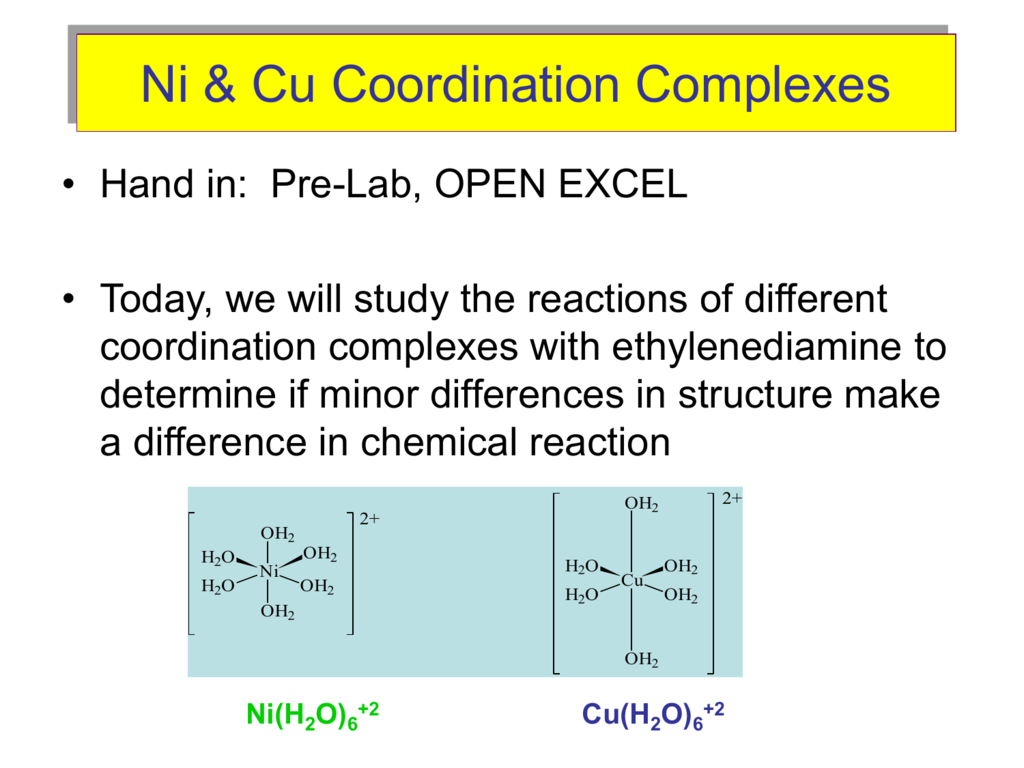
Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes
2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0277538713008310-fx1.jpg)
Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect

Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes
What is the hybridization, transition, spin, colour, magnetism, and geometry of [Ni(H2O) 4] +2? - Quora
![Electronic Structure of 3d [M(H2O)6]3+ Ions from ScIII to FeIII: A Quantum Mechanical Study Based on DFT Computations and Natural Bond Orbital Analyses | Inorganic Chemistry Electronic Structure of 3d [M(H2O)6]3+ Ions from ScIII to FeIII: A Quantum Mechanical Study Based on DFT Computations and Natural Bond Orbital Analyses | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/ic001258t/asset/images/medium/ic001258tf00002.gif)
Electronic Structure of 3d [M(H2O)6]3+ Ions from ScIII to FeIII: A Quantum Mechanical Study Based on DFT Computations and Natural Bond Orbital Analyses | Inorganic Chemistry
![Aqueous solution of Ni^2 + contains [Ni(H2O)6]^2 + and its magnetic moment is 2.83 BM . When ammonia is added in it, comment on the magnetic moment of solution. Aqueous solution of Ni^2 + contains [Ni(H2O)6]^2 + and its magnetic moment is 2.83 BM . When ammonia is added in it, comment on the magnetic moment of solution.](https://d1hj4to4g9ba46.cloudfront.net/questions/2015518_249889_ans_bbd2828107e04c46b63fdcb0f0c04801.jpg)

2 with gaseous NH3; crystal growth via in-situ solvation - ScienceDirect Reaction of [Ni(H2O)6](NO3)2 with gaseous NH3; crystal growth via in-situ solvation - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0022024814007805-gr3.jpg)

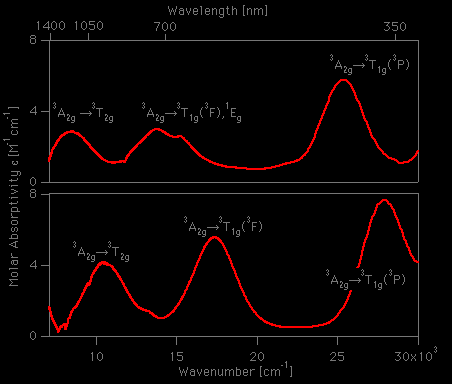
![How [Co(H2O) 6] 2+ is having higher crystal field splitting energy than [Ni( H2O) 6] 2+? - Quora How [Co(H2O) 6] 2+ is having higher crystal field splitting energy than [Ni( H2O) 6] 2+? - Quora](https://qph.cf2.quoracdn.net/main-qimg-fa42f87b83e5b7668101dfb77e274e3c.webp)
![A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain. A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain.](https://haygot.s3.amazonaws.com/questions/1955044_1567628_ans_f710a7dfc21d4ea7a5d920b41112c727.jpg)